Unmet Needs
3V Medical Research Group has, since inception, focused upon developing products that will meet unmet needs, both in the USA and globally.
Bacterial infections remain major causes of mortality and morbidity, especially in the Third World, while viral infections such as influenza or COVID-19 are responsible for mortality in all countries and consume healthcare resources on a scale that provides a constant global challenge.
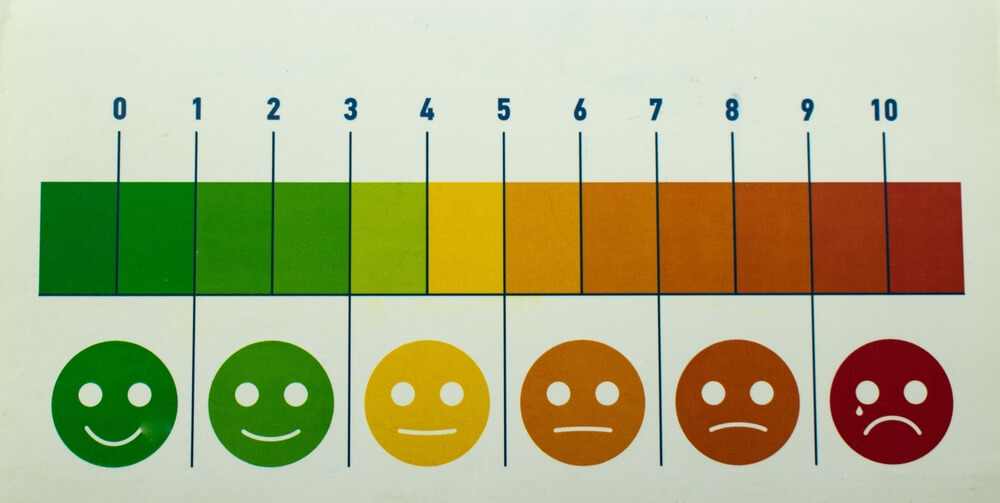
In the USA and other developed nations, pain associated with degenerative diseases of the elderly such as osteoarthritis or with chronic recurrent illnesses such as shingles or post-herpetic neuralgia, is a major cause of lost quality of life.
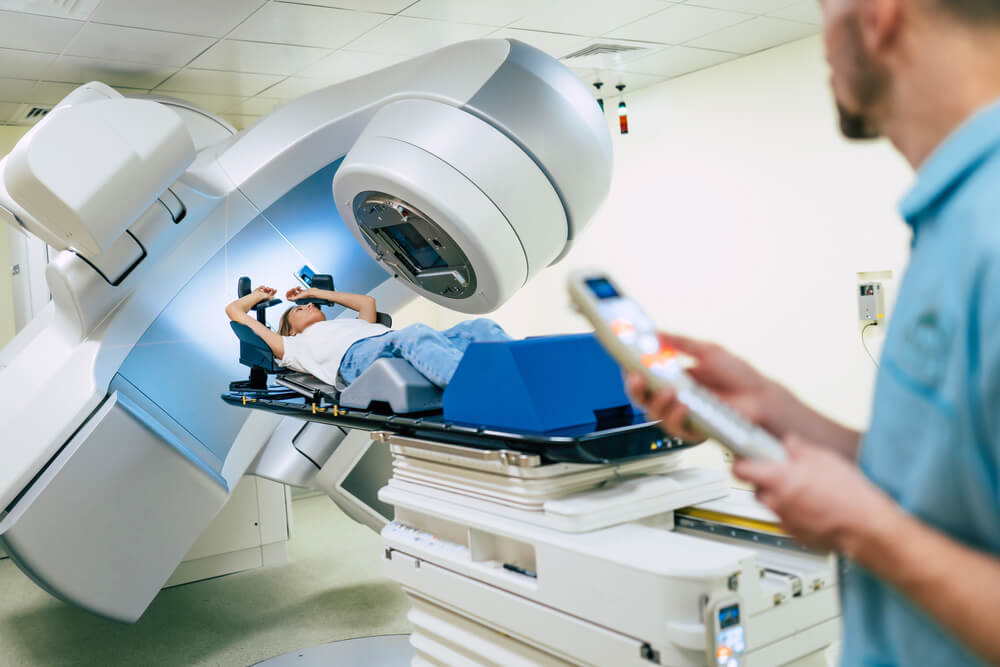
Severe discomfort associated with radiation dermatitis after radiotherapy for cancer, especially breast cancer, can be so severe as to lead on occasion to patients abandoning potentially life -saving treatment.
Pain and Discomfort
Osteoarthritis (OA) affects over 32 million Americans and 595 million people globally. Current oral analgesics have significant disadvantages: anti-inflammatory agents are only suitable for mild pain and may have significant toxicity in chronic use. Narcotics are potentially addictive and may impair skills such as driving.
Topical NSAIDs (non-steroidal anti-inflammatory agents) were developed to provide analgesia similar to their oral counterparts with less systemic exposure and fewer serious adverse events. However, in practice, severe cardiac, gastrointestinal and renal side effects restrict the prolonged use of NSAIDs.
Neither topical salicylates nor capsaicin have shown significant efficacy in the treatment of OA. In October 2007, diclofenac sodium 1% gel (Voltaren® Gel) became the first topical NSAID for OA therapy approved in the United States following a long history of use internationally. Clinical trial data suggest that diclofenac sodium 1% gel provides clinically meaningful analgesia in OA patients. However, diclofenac gel has significant associated adverse events, (AE) and the approved US label bears a “Black Box” Warning of cardiovascular and gastro-intestinal AE.
Ostrolux™ is under development to be a topical analgesic, indicated for the relief of the pain of osteoarthritis of joints amenable to topical treatment, such as the knees and those of the hands. It is expected to have efficacy comparable to topical or oral non-steroidal anti-inflammatory drugs (NSAIDs), with a significantly improved safety profile (in particular a lower incidence of cardiovascular and gastro-intestinal SAE).
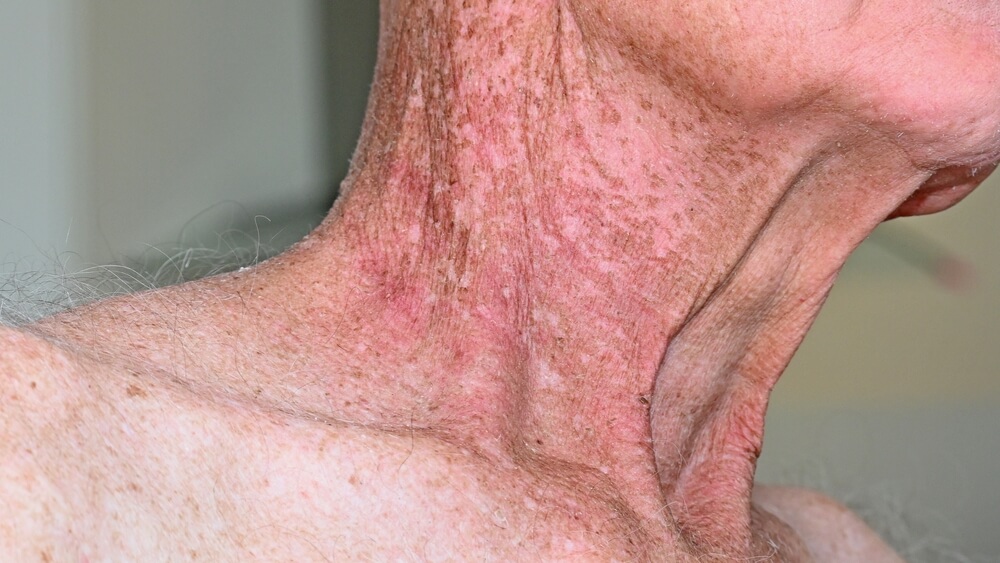
Radiation dermatitis (RD, also called radiodermatitis) is skin damage caused by radiotherapy (X-Rays) delivered during cancer management. The pathophysiology of radiation dermatitis is a combination of both the direct radiation injury and the subsequent inflammatory response and can occur at both the irradiation entrance and exit sites.
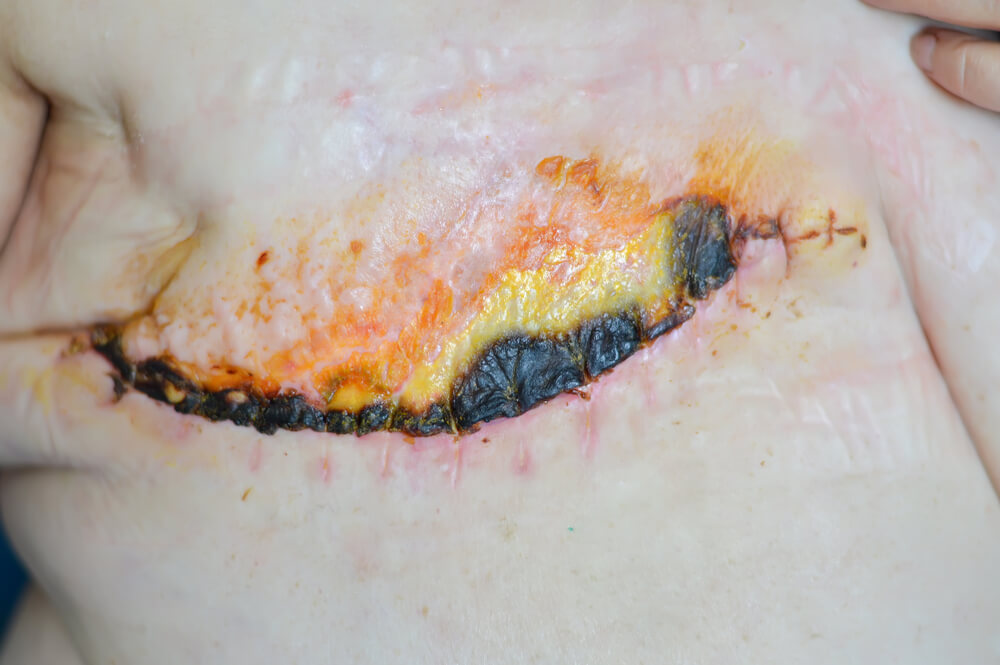
RD affects nearly 95% of patients who receive radiation for cancer treatment: ~70% of these patients have moderate-to-severe skin reactions. The skin reactions range from mild erythema (red rash) to dry desquamation (itchy, peeling skin) to more severe moist desquamation (open wound) and severe ulceration. Erythema is the first visible manifestation, occurring in more than 90% of these patients, followed by moist desquamation in more than 30% of patients
The more severe the skin damage, the greater the likelihood of infection. The pain and discomfort associated with RD may lead to the patient refusing further radiotherapy, thus reducing therapeutic effectiveness.
The management of RD has two major objectives: prevention of RD (prophylaxis) and treatment of RD. For RD prophylaxis, there are a wide variety of recommendations that include topical agents such as Aloe vera, gentle soap, petroleum-based ointments, topical corticosteroids, and sucralfate derivatives. When treating RD, physicians most often recommended bland emollients, anti-infectives such as silver sulfadiazine cream, and topical corticosteroids. There is no definitive evidence supporting any one intervention in preventing or treating radiation dermatitis. Under development, it is hoped that Ostrolux™ will be helpful in preventing or treating RD.
Reactivation of the chickenpox virus causes Herpes Zoster (HZ) or shingles and often leads to the recurrent prolonged symptoms of Post-Herpetic Neuralgia (PHN).
The incidence of HZ increases with age, with a peak occurrence in the 60-69 age group.
PHN can affect individuals of any age, but it is more commonly seen among individuals aged 50 years and above, especially those with a suppressed immune system.
Treatment options for HZ include antiviral drugs and analgesics. HZ / PHN can cause significant pain and discomfort, and it is crucial to address this aspect of patient care effectively.
Ostrolux™, with its potentially advantageous safety profile and possible effectiveness in pain control, represents a suitable candidate for HZ / PHN pain management.
Infection
The recent COVID pandemic has drawn attention to the role of infection as a major cause of mortality and morbidity. Causative agents, both bacteria and viruses, frequently enter the body via the nasopharynx, and would be amenable to prevention or treatment with an effective oropharyngeal spray or inhalation.
Some common infections that involve the upper respiratory tract, and would potentially be amenable to a spray or inhaler formulation of Luxsol™ include:
Copper has a well-established antimicrobial and antiviral effect. In February 2008, the US EPA approved the registration of 275 antimicrobial copper alloys, a number which expanded by 2011 to 355.
3V Medical Research Group has conducted several in-vitro studies of the antimicrobial efficacy of Luxsol™, including an in vitro evaluation of the efficacy of Luxsol™ in killing the COVID virus. Luxsol™ showed minimal or no toxicity in normal cells. However, it demonstrated antiviral activity against COVID virus.
Luxsol™ has also demonstrated efficacy in killing Strep pneumoniae, Hemophilus influenzae, Human Immuno-deficiency Type 1 virus, and multiple other pathogens that are not predominantly transmitted via the upper respiratory tract.